The water treatment process to deliver safe and wholesome water to customers includes many steps. Coagulation, flocculation, sedimentation, filtration, and disinfection are the water treatment processes that make up a conventional surface water treatment plant. These water treatment processes ensure that the water consumers receive is safe to drink and aesthetically pleasing.
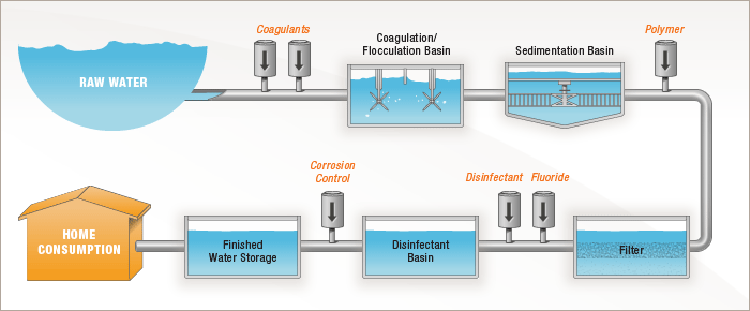
Water systems that operate a surface water treatment plant must meet the Surface Water Treatment Rule (SWTR). The goal of the SWTR is to reduce illnesses related to pathogens in drinking water. These pathogens include coliform, Giardia, and Cryptosporidium. Meeting the SWTR requires a multi-barrier approach to treatment. This means that the treatment plant must both remove and inactivate pathogens. The water treatment process of coagulation, flocculation, sedimentation, and filtration remove the pathogens. The disinfection water treatment process inactivates them.
The small particles in water may consist of silt and clay, color bodies, precipitated iron or manganese oxides, and even bacteria and algae. Together, these particles make the water appear cloudy. This cloudiness is known as turbidity. Visual turbidity is unpleasant to consumers. Visual turbidity is also an indicator to operators and regulators that the water may still contain pathogens. The Surface Water Treatment Rule therefore requires that turbidity be removed to very low levels. The process to remove that turbidity begins with coagulation.

Coagulation
Coagulation is defined as the water treatment process of increasing the tendency of small particles to attach to one another and to attach to surfaces such as the grains of a filter bed. Many surface water supplies contain particles that are too small to settle out of solution on their own. These small particles often carry a small negative charge which make the particles repel each other like similar ends of a magnet. Water system operators must use chemicals in order to neutralize these small charges, help the particles attach to one another, and become heavy enough to settle out of solution.
A coagulant chemical is injected to neutralize these small negative charges and then the water is rapidly mixed. The rapid mixing disperses the coagulant and also increases the interaction of these small particles.
History of Coagulation in Drinking Water Treatment
Coagulation has been an important process in high-rate filtration plants in the United States since the 1880s. Aluminum and iron salts have been used in the coagulation process since the beginning. These salts are still the most commonly used coagulants today. Common coagulants used today include aluminum sulphate (alum), ferric sulphate, ferric chloride, and sodium aluminate. Synthetic organic polymers were introduced in the 1960s. Depending on your system’s water quality, it may be necessary to employ a combination of two or more coagulants.
Aluminum and ferric coagulants put a large number of positive ions in the water. These ions begin to form flocs, which then attract the negatively charged particles in the water. As the particles collide in the mixing area they begin to stick together a form larger and larger flocs. Temperature, pH, alkalinity, and the amount of turbidity in the water control the reactions of aluminum and ferric salts in the water.
Factors Effecting Coagulation Process
The water’s pH has a large impact on the coagulants effectiveness during the destabilization process. The optimal pH range for coagulation is 6 to 7 when using alum, and 5.5 to 6.5 when using iron. A large amount of coagulant may be required to lower the pH into the optimal range for high alkalinity waters. This results in high chemical costs and large amounts of sludge produced in the treatment process. It therefore may be cost effective to add acid in addition to the coagulant to bring the pH down and reduce the amount of coagulant required in this situation.
Water temperature also impacts the coagulation process because it effects the viscosity of water. Both alum and ferric salts form flocs at a slower rate as the water temperature decreases.
Flocculation
Following the coagulant chemical addition and the rapid mix processes, the raw water will continue on to a flocculation basin. The goal of the flocculation treatment process is to increase the size of the flocs in order to increase their ability to settle out.
Flocculation Basin Design and Short Circuiting
Flocculation basins are normally designed with baffles to decrease the chance of short-circuiting. Short circuiting occurs when water is able to flow almost directly from the inlet to the outlet of a tank or treatment process without receiving adequate contact, settling, or reaction times. The baffles usually separate the flocculation basin into a few distinct compartments. These compartments make it easier for operators to control the amount of residence time and mixing energy the water receives.
Flocculation Basin Operations
Inside the flocculation basin are typically large, very slow-moving paddle mixers that promote the interaction of particles. The compartments nearer to the tank inlet will typically receive higher mixing energies to increase the interaction of particles. Nearer to the tank outlet, the flocs become larger and thus more fragile. The water here will receive less mixing energy in order to not break apart the flocs but still keep them moving so they don’t settle out prematurely.
If you walk from one end of the flocculation tank to the other, you can see these particles increase in size as more and more particle collisions occur. This process of particle size growth is referred to as agglomeration.
Chemicals Used During Flocculation
In order to enhance floc formation and increase the strength of the floc structure, some systems may add a flocculant to the water. Larger flocs will be able to settle out of the water more quickly further down the water treatment process chain. You may see the terms “flocculant”, “coagulant aid”, or “filter aid” used in books or plant operation manuals. All of the terms refer to roughly the same thing: a chemical added to the water to increase the size and stability of the flocs formed.
Sedimentation (or Clarification)
The water continues on to the sedimentation basin, or clarifier, after the flocs have been formed. The goal of this stage of the treatment process is to reduce the amount of solids in the water before the water is filtered in the next treatment step.
The large flocs will settle out of suspension via gravity. Clarifiers can remove a very large percentage of the suspended materials in water. In some plants, clarifiers remove as much as 90% of the suspended solids load. Particles that do not settle will be removed by filtration in the next treatment step.
Sedimentation Basin Design

Clarifiers are designed and built with great care to ensure that the water flows through them very slowly. The basins are typically rectangular or circular. In rectangular basins, the water will flow from one end to the other and the water velocity will be parallel to the basin length. In circular basins the settled water flows from the center of the basin to the outside.
Inclined Plate or Tube Settlers
Installing inclined plates or tubes to the clarifier can greatly enhance its ability to settle. Adding inclined tubes or plates also increases the effective surface area of the basin, and decreases the depth that a particle must travel in order to settle out.
Sedimentation Basin Operation
Sedimentation basins are designed and operated with two key parameters in mind: detention time and overflow rate. Detention time is defined as the volume of the basin divided by the flow through the basin. The volume is expressed as cubic feet, and the flow as cubic feet per minute. Detention time is the theoretical amount of time that a quantity of water would take to pass through the basin and exit the outlet. Most clarifiers at conventional surface water treatment plants are designed to provide a theoretical detention time of around four hours. As discussed above, tanks and clarifiers normally experience some form of short-circuiting. Therefore, detention time is the average amount of time for water to flow through the tank, and not the exact time.
Surface overflow rate (SOR) is another important parameter in the design and operation of clarifiers. Surface overflow rate is defined as the flow through the clarifier (Q), divided by the surface area of the clarifier. Flow is expressed in units of gallons per minute, and the surface area is in square feet.
Clarifiers are typically operated at a SOR of 0.5 gpm/ft2. By operating at this low rate, the flocs are provided enough time to settle out via gravity. This reduces the suspended solids load on the filters, which will in turn increase the filter run times and the decrease the chance for a turbidity breakthrough. By installing inclined plate or tube settlers, as discussed above, the effective surface area of the clarifier will increase. This allows plants to increase the flow through the clarifier and treat more water per day. Clarifiers equipped with inclined tube settlers can have SOR as high as 2.0 gpm/ft2.
Sludge Handling
The particles that settle out are known as sludge and must be regularly removed from the clarification tank. Clarifiers will normally be equipped with rakes to capture the sludge. Some plants may try to dewater the sludge to collect some of the raw water. This captured water will be plumbed back to the head of the treatment plant in order to go through the whole treatment process again.
Filtration
The final water treatment process in removing particulates is filtration. The sedimentation process will have already removed a large percentage of the suspended solids. Sedimentation is unable to remove many small particles in water though. Filtration will remove these microorganisms and other suspended material that did not settle out previously.
Single Media, Dual Media, Mixed Media Filters
Filters are typically classified based on the type of media used. There are single-media, dual-media, and multi-media filters. Single media filters are typically just sand, and are not common in modern treatment plants. Dual-media filters are the most common types of filters. Anthracite coal and sand are the typical filter materials in dual-media filters. Anthracite coal, sand, and garnet sand are the typical components in a multi-media filter. A single-media sand filter is expected to remove particles from 25-50 microns in size, and a dual-media filter can be expected to remove particles down to 10-25 microns.
Filter Ripening
A filter must be ripened when it is first placed into service or following a backwash. If the filter media is too clean, and the pore spaces between the sand grains and the anthracite coal too large, there are chances for particulates to pass right through the filter. The pore spaces decrease and the filter is better at removing the particulates as more water is filtered and more particles are captured. Until the filter is ripened, the water produced by it may not meet the necessary turbidity requirements. Systems must be equipped with the ability to pump this non compliant water to waste without it entering the distribution system.
Filter Operations
Filters operate at a filter loading rate that is based on the water quality and filter media used. Typical filter loading rates range from 2 to 4 gpm/square foot. The filter loading rate is a critical parameter in the operation of the treatment plant because it determines the water velocity through the filter and the filter run times. Filter run time is the length of time that a filter can be in production before it has to be backwashed. Filter run time can be seen as an indicator of how effective the prior steps of treatment were in reducing the solids load on the filter.
The very top portion of the filter typically removes the majority of the particles in the water. This is because the filter media stratifies relative to their mass. So the heavier sand will be on the bottom of the filter and the lighter anthracite coal will be on the top of the filter. Particle removal happens only when the particles make contact with the surface of the filter media. The pore spaces of the anthracite coal are very small, which means that the particulates in the water cannot pass through. More and more particulates are captured in the filter as more water passes through it. Eventually the filter must be backwashed because it becomes so loaded with particles.
Backwashing a Filter
Backwashing the filters is used to dislodge and remove particles trapped within the filter bed, to reduce head loss across the filter, and to keep the filter media clean. If a filter is ran too long without being backwashed, it can become overloaded and have a turbidity “dump”. This occurs when the pressure builds up so high on the filter that a channel develops in the media and allows particles to flow right through.
The goal of the backwashing process is to remove the captured particulates without removing the filter media. The flow of water is reversed through the filter during the backwash. The reversed flow of water expands the media and washes the captured particulates off of the media. It is important to not overly wash the media. The ripening time of the filter can be reduced and the filter can be placed back into service more quickly by leaving some particulates in the filter. It is important that plants develop specific procedures for the backwash process so all operators perform the backwash in the same way.
Each plant will have their own parameters of when to initiate a backwash. A filter may be backwashed when the head loss, which is the pressure build up, reaches a certain level. The system’s domestic water supply permit may also specify that a filter be backwashed following a certain length of time, regardless if that target head loss is achieved.
Disinfection
As discussed previously, the surface water treatment rule requires both the filtration and disinfection of surface water sources. The water must be disinfected now that it has been filtered.

Meeting the Chlorine Demand and Maintaining a Residual
The primary function of disinfection is to reduce the number of pathogens in the water. Water can be thought to have a natural chlorine “demand”. This means that when you add chlorine to the water, things other than the pathogens you are targeting will react with and consume some of the chlorine. This includes both inorganic material (iron, manganese, ammonia, etc.) and other organic material.
Water systems that treat surface water must also carry a disinfection residual in their distribution system. So enough disinfectant must be added to the water to meet the natural chlorine “demand”, inactivate the targeted pathogens, and still carry an acceptable residual into the distribution system.
Disinfection Contact Time
In order for systems to be sure that they are properly disinfecting the filtered water, the Surface Water Treatment Rule requires systems to provide enough contact time. Contact time (CT) is a function of the known disinfection concentration and the amount of time that the disinfectant is in contact with the water. Contact time is expressed in terms of mg/L-min. The EPA has published tables that show how much CT credit water systems will receive. In order to use these tables you use the concentration of chlorine, time, water temperature and pH.
Although CT is the most important operational factor in inactivating pathogens, if you make your chlorine dose too high it might lead to unexpected consequences. As the EPA explains in the Drinking Water Treatability Database, a high chlorine dose may lead to taste and odor issues, and possibly disinfection byproducts. Disinfection byproducts are formed when chlorine reacts with natural organic material in the water. Disinfection byproducts introduce their own operational challenges and regulations that must be dealt with.
History of Chlorination
Disinfection using chlorine has existed for more than a century. Chlorine disinfection was used to eradicate many of the water-borne diseases that plagued humans for centuries. This includes both typhoid and cholera. Chlorine disinfection is one of the most important public health accomplishments of all time and has saved countless lives.
Chlorination Operations
Chlorination was one of the first drinking water disinfection methods. It is still the most commonly used disinfection method used today. The filtered water is injected with either liquid sodium hypochlorite, gaseous chlorine, or solid calcium hypochlorite. Chlorine is a strong oxidant. It is used to both disinfect and also to remove color, taste and odor compounds, iron and manganese, and other dissolved inorganic contaminants such as arsenic.
Alternatives to Chlorine
Chlorination is an effective disinfectant against viruses and Giardia lamblia. In recent years, hardier organisms such as Crytosporidium have emerged that require a high CT to inactivate. Alternative disinfectants to combat these hardier organisms have been developed. Ozonation and UV light are two alternative disinfectants that work very well against Cryptosporidium. Each of these have their own set of unique challenges that must be taken into account to operate effectively. The Surface Water Treatment Rule requires that systems maintain a detectable residual in the distribution system. Neither of these alternative disinfectants provide a residual. So prior to the water entering the distribution system it must have chlorine added to it.
Conclusion

In order to meet the requirements of the Surface Water Treatment Rule, a water system must both remove and inactivate the pathogens in the water. This process begins with coagulation, which destabilizes the particles in the water. Then, during flocculation, the destabilized particles bump into each other and form larger and larger flocs. These large flocs are given adequate time to settle out of solution via gravity during sedimentation. Any remaining particles and pathogens will be removed during the filtration treatment process. Finally, the water is disinfected to inactivate any remaining pathogens prior to entering the water system’s distribution system.
By implementing these treatment processes and meeting the Surface Water Treatment Rule, the incidence of water borne disease outbreaks have become a very rare event in industrialized nations and countless lives have been saved.